In product manufacturing, one of the most important processes ideals for product durability and aesthetics is the anodizing process. The process is ideal for several materials, however, the most important and commonly used one is aluminum. As a beginner in machining, you might not be familiar with anodizing, talkless of how to anodize aluminum.
Therefore, in this guide, we’ll take you through everything you need to know about anodizing aluminum. This will be in form of looking at the what, how to anodize aluminum and the reasons for aluminum anodization.
What is Anodized Aluminum?
Anodizing is a common finishing method used with a selection of nonferrous metal materials. This includes aluminum, titanium, and magnesium.
It involves an electrochemical process that transforms the outer surface of metal parts into a durable and highly corrosion and scratch-resistant layer. The process is also highly decorative. On application, it offers a shiny finish that comes in a variety of colors.
How Does Aluminum Anodizing Work?
Before knowing how to anodize aluminum, the first thing to know is how anodizing works. Almost any aluminum part can be anodized. Whether it has been CNC machined or made using sheet metal fabrication. The process, which may seem complex due to the various electrochemical reactions taking place, is quite simple and cost-effective. Therefore, anodization is a popular choice across many industries.
Steps of Anodizing Aluminum
Anodizing again might look complex, however, the steps are straightforward. Below are the general steps used in the anodizing process.
- Step 1: First, cleaning of the aluminum part occurs before undergoing anodization. This is important for the removal of impurities that can hinder the process.
- Step 2: The placement of the material into a bath of electrolytic solution occurs and there is the application of a direct electric current. This creates a positive electric charge in the aluminum and a negative charge in the solution’s electrolyte plates. The resulting electrochemical reaction creates pores on the surface of the aluminum component, which enable the aluminum substrate to bond with the negatively charged O2 ions in the solution to create aluminum oxide. This step answers the question “what does anodized mean.”
Consequently, it results in the creation of an anodic oxide barrier layer on the part’s surface. This barrier layer is more resistant and durable than the underlying aluminum.
Materials for the Anodizing Process
Based on its principle, anodizing is only available for conductive materials like metals. However, it does not mean that aluminum is the only option. Anodized metals also include magnesium and titanium.
Other questions related to the available materials for the anodizing process include whether it is possible to anodize steel or stainless steel. However, this is not possible. The reason is due to the formation of iron oxide (rust) on the steel. The iron oxide (rust) formed does not form a tight, tenacious, corrosion-resistant coating on steel. Therefore, it can’t be beneficially anodized.
Different Types of Anodizing Aluminum Process
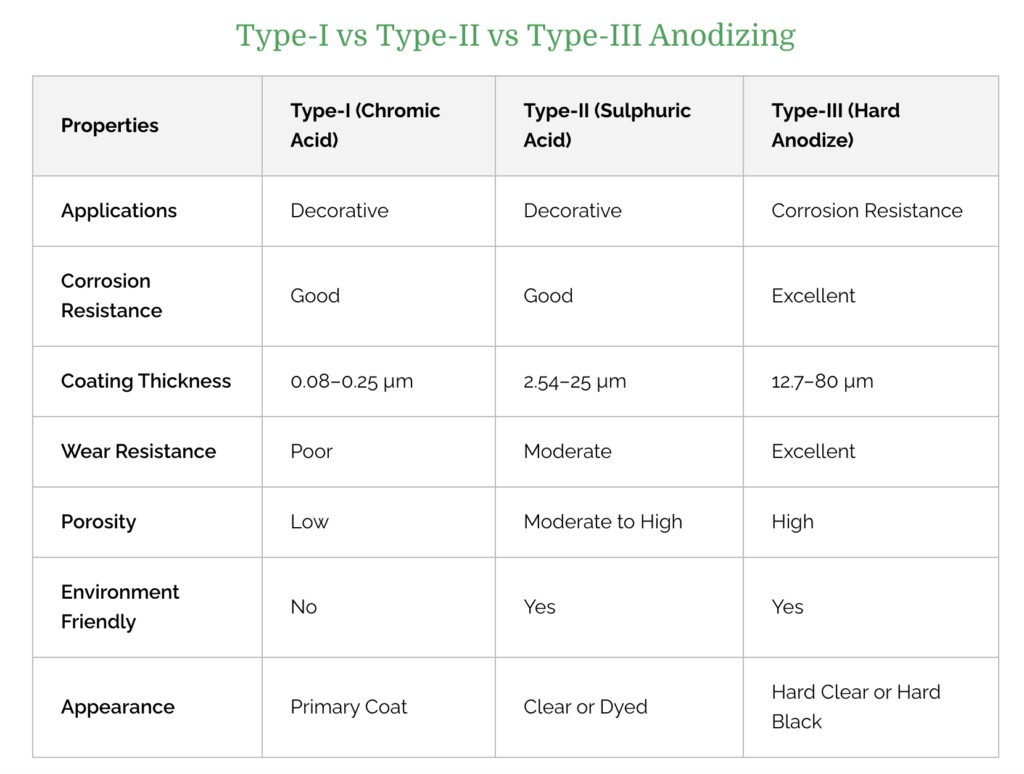
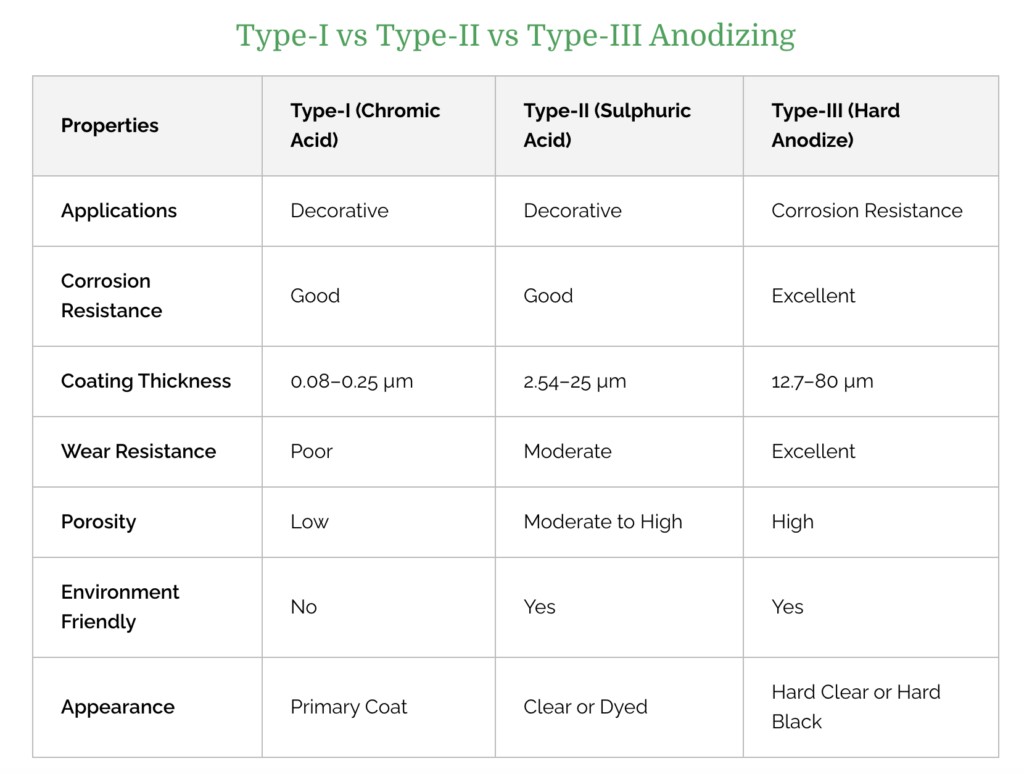
To know how to anodize aluminum, there is a need to know the types of aluminum used in the anodizing process. There are three commonly used types of anodizing process and each type of process results in a different set of functional and aesthetic properties.

Type I – Chromic Acid Anodize
Type I anodizing process uses chromic acid to create a thin coating on the surface of metal parts (up to 0.0001 inches).
Though Type I is the thinnest anodizing coating, it still results in parts with increased corrosion resistance. However, it also results in the least color absorption when dyed.
Type II – Sulfuric Acid Anodize
Type II anodizing process uses sulfuric acid instead of chromic acid. This results in a slightly thicker surface layer on the aluminum part.
Type II Sulfuric acid anodize has a thickness of 0.0002 to 0.001 inches and is better suited for dyeing parts.
Type III – Hardcoat Anodize
This is often called “hard anodizing”, which also uses a sulfuric acid approach. However, it results in a thicker (> 0.001 inches) anodized layer when compared to Type II.
Hardcoat anodized parts have the best abrasion resistance and color dyeing potential. However, it may not be ideal for parts with extremely tight tolerances.
Which Type of Anodizing Should You Choose? What’s the Difference?
Considering the different applications of aluminum parts, deciding which type of anodizing process is quite an important step. Here are some types on how to quickly compare the types and use them for your reference.
- Type I uses chromic acid to create a thin coating on the surface of metal parts. It is ideal where there is a need for where corrosion resistance for example in making aircraft parts.
- Type II anodizing uses sulfuric acid to create a slightly thicker surface layer on the aluminum part. Its application is in making finishing consumer good products, aircraft components, architectural parts, and kitchenware.
- Type III is like Type II but results in a thicker corrosion resistant layer. This makes it well suited for parts that must withstand extreme temperatures and chemical exposure. For instance, the military use it in producing robust metal parts.
It won’t be surprising if you cannot decide which type of anodizing is more suitable for your project. This is where we at RapidDirect can help as we can easily remove such trouble by providing professional suggestions based on your project. You can upload your design files and get in touch with us.
What Are the Color Options for Anodized Aluminum?
Another thing to note on how to anodize aluminum is the color options available. This is because one of the most significant advantages of anodizing is the various color options available. The standard anodizing aluminum colors include clear, bronze, champagne, and black. At RapidDirect, you have access to color cards with Pantone numbers from which you can decide the color you want.

Advantages Of Anodized Aluminum
Aluminum is a broadly used material because of its beneficial properties. Although it does not rust, it is still susceptible to other conditions. For example, it can undergo wear and tear, due to oxygen exposure. Here, we’ll dive into the benefits of using the anodizing process on aluminum parts.
· Improve Material Properties
First, the finishing process significantly improves material properties on the part’s surface. This includes increasing corrosion, scratch, and weather resistance. Further, because the process is electrochemical, the barrier layer created using anodizing becomes a part of the component. This means that it can’t peel or chip like paint coatings.
· Improved Insulating Property
Also, the outer anodized layer of a part has insulating properties. This means that parts may have lower electrical conductivity than before.
· Better Surface Effect
Another reason that many customers choose to anodize their aluminum products is aesthetics. Anodizing can also apply a color finish to metal parts and there are practically infinite colors to choose from. This includes clear anodized aluminum, black anodized aluminum, blue, gold, grey, red, etc.
Design Tips for Anodizing Aluminum
Learning how to anodize aluminum parts might not be complex. However, some tips will help smoothen the process especially if you are a beginner. Below are a few important tips that you can use in the process.
· Watch Out for Tolerances
If you know you want to apply the anodizing process to your aluminum component, be aware that the process does add some thickness to the part. This is because it can (albeit marginally) affect part tolerances.
If tight tolerances are critical, consider the option for Type I or Type II anodizing. You can also take the extra layer into account in the design stage.
· Edges And Corners
With the anodizing process, an important design tip is to ensure that all edges and corners of the workpiece have radii of at least 0.5 mm. Part designs should also not integrate any burrs.
The reason for these design considerations is that they help to prevent overheating (and even burning) of the workpiece due to a high concentration of electric current.
· Consider Using Other Finishing Steps
Anodizing is an electrochemical process. Therefore, it doesn’t have the same effect as bead blasting or polishing. If an aluminum machined part goes straight to anodizing, some machine marks or scratches will likely remain on the finished part’s surface.
For this reason, if you require a completely uniform surface finish, it can be beneficial to use polishing, bead blasting or another mechanical finishing process beforehand. Anodizing will make the surface of a part smoother than before.
· Work With Batches
If you are coloring your aluminum parts or products, it is advisable to anodize them in small batches. This ensures a greater degree of color uniformity, as it can be difficult to exactly match a color from one batch to the next. The ideal scenario for color consistency is to anodize a small batch of small parts at once.
If you’re interested in the cost of anodizing aluminum, you can learn a lot from this article.
Applications for Anodizing Aluminum

Anodizing is a high-quality and affordable finishing process. Therefore, it is popular and used for a few applications across a wide variety of industries. So broad is its use that you likely encounter an anodized metal part in your day.
Some of the industries that use anodizing regularly are:
- Aerospace.
- Automotive.
- Architecture.
- Consumer goods.
- homeware sectors.
While it is impossible to list all the specific applications that use anodized aluminum, here are a few that many people will relate to:
- Kitchen equipment.
- Duct covers.
- Light fixtures.
- Food preparation products.
- Photo equipment.
- Radio equipment.
- Electronic casings.
If you need anodized aluminum parts, you can contact RapidDirect and upload your files to get an quote.
How to Identify if the Anodizing Process is Successful?

There are many ways to tell if a part has undergone anodizing. Below are some useful ways you can employ.
- Check For Matte Finish
You can usually tell by the matte finish that anodizing creates.
- Use a Simple Scratch Test
Scrape a coin on the surface of the aluminum part. If a scratch is visible, the part has likely just been polished, not anodized. An anodized part will be scratch-resistant.
- Color Dispersal
A good anodizing process will result in a uniform surface with even color dispersal. Anodizing defects to watch out for on your finished product include anodizing burns caused by high current densities and not enough agitation in the anodizing process.
Conclusion
The anodizing process is important in product manufacturing, and to answer this question, this article discussed what is anodized aluminum, how to anodize aluminum and other tips you need.
At RapidDirect, anodizing is one of our integral finishing solutions for metal parts, along with bead blasting, brushing, polishing, electroplating, powder coating and painting. Our expert team is highly knowledgeable in the anodizing process and guarantees high-quality aluminum parts to our customers. To see if anodizing is the best finishing solution for your part or product, or a quote, just get in touch with a member of the RapidDirect team. We’re at your service!
FAQ
One of the reasons anodizing is a popular finishing process is that it is highly cost effective. However, the cost of the process is dependent on several factors. This includes part quantity, part dimension and shape, anodizing type (i.e., coating thickness) and color.
In short, dying a complex part will cost more to anodize than a simple part with no color finish. Please get in touch with us at RapidDirect for a customer-specific anodizing quote.
The anodizing process creates a barrier layer on the surface of aluminum parts bonded on a molecular level. This means that it cannot peel or chip away, unlike paint coatings. A properly anodized part should not be worn for many decades.
Similarly, dyed anodized parts that are properly sealed should not fade for at least five years, often more. You should also note that the thicker the anodized layer (Type III being the thickest), the less wear the part will experience.