Electroplating is one finishing process that has applications in a wide range of industries. This process improves parts’ appearance and properties. Initially, metals can only be electroplated with other metals, but with the recent technological advancement, improving non-metals with this process is available too.
Besides, electroplating can combine the desirable properties of certain metals with other materials. These properties often include strength, abrasion, appearance, corrosion resistance, and electrical conductivity. Moreover, this process aims to boost or improve the material’s properties. The material could be metals, plastic, or even wood.
Aside from this information above, how does electroplating work? What metal materials are ideal for use in this process? What are the advantages and disadvantages of this plating process? Keep reading as we provide answers to these questions and other important things you need to know about the electroplating process.
What Is Electroplating?

Electroplating is a coating process that has been around since the early 19th century. Although there has been advancement in the technology used, the basic process remains the same.
Electroplating simply means coating an object or a material with a metallic layer or layers using electric current. This process also termed electrodeposition, results in the deposition of a thin layer of metal onto a material. Consequently, this process aims to alter the material’s physical properties, also called the substrate.
Additionally, due to the electroplating process, the substrate has better aesthetic appeal, corrosion protection, and increased resistance to wear and tear or increased thickness.
How Does Electroplating Work?

Electroplating works by dissolving and depositing a metal onto another surface through an electric current. There are four primary components of this process.
- Anode: This is the positively charged electrode used in the circuit. The anode holds the metal used for the plating process.
- Cathode: This is the negatively charged electrode used in the circuit. It holds the material you want to plate, also called the substrate.
- Plating Solution: This is one of the most important metal finishing solutions. It serves as a catalyst facilitating the flow of electricity in the circuit. The plating solution usually contains copper sulfate and one or more metal salts.
- Power Source: The power supply adds current to the circuit. The power source introduces electricity to the system when connected to the anode.
Electroplating Working Principle
So, how is electroplating done? Below are the steps of electroplating.
Place the anode (metal) and cathode (substrate) in the plating solution or electrolyte. Subsequently, introduce electricity to the setup through the anode.
Consequently, on the introduction of electricity to the anode, oxidation occurs. The result forms the dissolution of metal atoms in the plating solution as positive ions (cations). Furthermore, the current in the circuit causes the movement of the metal ions (positively charged ions) to the negatively charged substrate. This results in the deposition of a thin metal layer on the substrate.
Also, for the electroplating process to be successful, always remember the following points. Firstly, the electric current’s quality affects the plating process Quality here includes the voltage level and electric current application time.
Secondly, the chemical composition of the electrolyte and its temperature also determine the effectiveness of the process. Lastly, always consider the position of the anode to the cathode. This is because the distance the dissolved metal ions travel to get to the substrate also determines how effective the plating would be.
However, for plating to be optimal, engineers have to adhere to these precautions.
Precautions In The Electroplating Process
- Handle the solution with great care since it contains sulfuric acid and is highly corrosive. If the solution or electrolyte gets into your eyes, rinse your eyes with a lot of water, and call a doctor immediately.
- Always wear safety gadgets like goggles and gloves when carrying out this process to avoid direct contact between electrolytes and skin. Other important gear to have includes an apron and fume hood. Ensure you use the fume hood, as it prevents electrolyte fumes from reaching your face on their way out.
- Also, keep the plating solutions out of the reach of animals and young children.
- Avoid contact between electrolytes and household chemicals. Contact between them could trigger a reaction that could either render the electrolyte useless or release hazardous gasses into the atmosphere.
- The ideal temperature for storing electrolytes is between 40 to 95 degrees Fahrenheit. Furthermore, always store it in the original container with the lid tightly sealed.
- Always use a plastic funnel when transferring the electrolyte from the electroplating kit.
- Follow all electrical safety precautions when attempting this process. These include rubber mats, grounding, fusing, and insulated gloves.
- Do not wear rings and other jewelry during the process as they are often metallic and could cause electrocution.
3 Types of Electroplating Methods
There are different types of electroplating methods to employ when coating a substrate. These include:
- Barrel Plating
- Rack Plating
- Reel to Reel Plating
Let us discuss these methods in more detail.
Barrel Plating
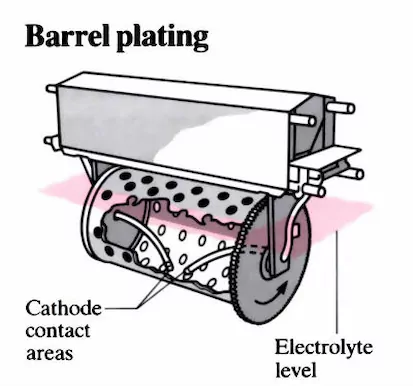
This rotation causes the substrate within the barrel to tumble, facilitating the uniform application of the coating metal. Consequently, engineers use this type to achieve enhanced corrosion resistance and improved substrate appearance. In like manner, engineers also use barrel plating to improve a substrate’s resistance to wear and tear.
Also, since it allows the addition of more than one substrate inside the barrel, it provides a cheaper means of plating high-volume parts. This makes barrel plating ideal for plating fasteners and stampings. On the other hand, plating with this method takes more time since it requires a low electrical current.
Rack Plating

Rack plating differs from barrel plating. In this case, substrates stay in one place. This method does not allow the freedom of movement seen in barrel plating. Also, it involves using metal racks with the substrates affixed to the racks with spring fingers, wires, or screws. As a result, the substrate remains immobile on immersion into the electrolyte.
Besides, it is important to note that engineers employ rack plating when working with delicate parts. In other words, parts might find it difficult to withstand the tumbling experience of barrel plating.
Additionally, rack plating is also ideal for use when the substrate is large or complex. It is the engineer’s choice when a high-quality finish is important, giving it application in medical and electronics, automotive as well as defense and military gadgets.
On the other hand, rack plating requires a lot of labor input for success, so it is quite expensive. Nonetheless, this plating method guarantees protection against damage to substrates during the plating process.
Reel to Reel Plating
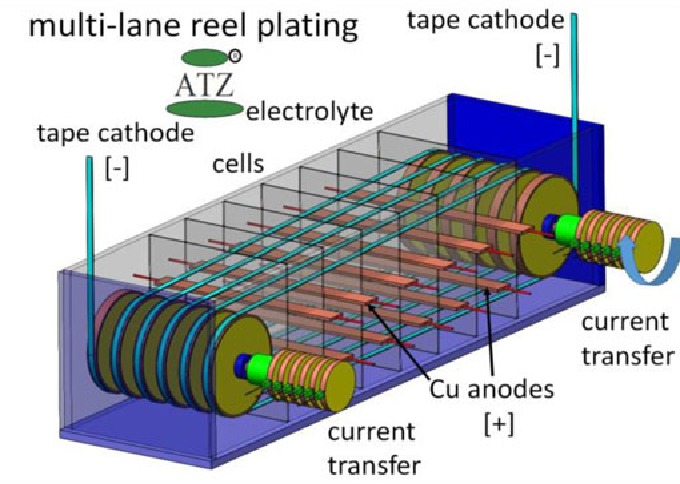
Reel to reel plating is both economical and efficient. It is a unique plating method because it allows the selective deposition of metals on a substrate. Often used for plating strips of manufactured or stamped products, reel-to-reel plating offers engineers more benefits than other plating types.
There are different types of reel-to-reel plating;
- Controlled Depth Plating: Plating occurs only on both edges of the substrate. It does not occur anywhere else.
- Spot Plating: Using this type of reel-to-reel plating involves using a mask. Here, metal deposit only occurs in unmasked areas in a spot pattern.
- Flat Stock: This method is similar to spot plating as it involves the deposition of metal on unmasked areas of a substrate. The only difference between them is the absence of the spot pattern as seen on spot plating.
- Tape Masking plating: This reel-to-reel plating method involves metal deposition on a substrate in a continuous strip. It is important to note that you should cover areas that do not require plating using masking tape.
Generally, reel-to-reel plating is ideal for use when you want to plate a particular substrate area. Furthermore, it has a high plating rate and offers increased conductivity and durability to substrates. Materials plated using this method are also durable and resistant to corrosion. Besides, this plating method, when used on high-volume jobs, also saves costs.
Metal Materials Suitable For Electroplating
The electroplating process can occur with either one metal or a combination of metals. There are many metals that engineers use for this process. However, below are the most common metals used.
- Copper: Copper electroplating boosts adhesion between material layers. It also increases the heat resistance and conductivity of a substrate.
- Zinc: Zinc has high corrosion resistance. Furthermore, when electroplating occurs using zinc alloyed with nickel, it improves the substrate’s resistance to atmospheric corrosion.
- Nickel: This is one of the metal materials suitable for electroplating because it is wear resistant. It also has alloys that offer elemental resistance, conductivity, and hardness substrates.
- Silver: Silver metal plating finishes have high ductility and malleability. It also has a pleasant appearance and resists contact wear excellently. Furthermore, silver improves a material’s electrical and thermal conductivity.
- Palladium: Often used because of its corrosion resistance, palladium also improves the substrate’s hardness and resistance to corrosion.
- Gold: This is a precious metal with high aesthetic appeal. Moreover, it imbues substrates with high conductivity, tarnishes, corrosion, and wear resistance when used.
- Tin: This bright metal is inexpensive and environmentally friendly. It makes substrates corrosion-resistant and highly malleable when used.
Advantages and Disadvantages of Electroplating
Now that we know more about electroplating and why it is important to engineers and manufacturers, let’s discuss its merits and demerits.
Advantages of Electroplating
- Offers Substrate material protection: Protecting objects from corrosion and tarnishing is one of the major advantages. Furthermore, it also improves object shock protection and heat resistance.
- Reduces friction: Electroplating objects minimizes the friction on metals when rubbed together. Hence, reducing scraping and heat generated. Moreover, less friction also translates to less wear and tear, allowing you to use objects for a long period.
- Improving object properties: this process imbues objects with extra properties such as thickness, magnetism, and conductivity. This gives the process application in producing electronics and other products that require materials with such properties.
- Improved adhesion: In some cases, the electroplated surface is not the final coat but just an intermediate step. The surface acts as a glue, holding the base material and the outer coating together, improving adhesion.
Disadvantages of Electroplating
- Environmental Pollution: When not properly done, this process can produce hazardous waste that is detrimental to the environment. However, you can avoid this with proper waste management.
- Expensive to set up: A complete setup for this process is quite expensive as you would have to get metals, chemicals, and other expensive equipment before it is up and running.
- Takes time: Metal deposition occurs very slowly, which takes a lot of time. It even consumes more time when the material requires more than one layer.
Applications of Electroplated Parts
Electroplating has applications in a wide range of industries. Let’s examine a few of them.
Aerospace
Aircraft components are often subject to a wide variation in temperature, so plating them helps increase their lifespan. Besides, it improves base metal’s resistance to wear and tear.
Automotive
To achieve an aesthetic finish, automotive companies apply electroplating metals such as chrome and nickel to various car and motorcycle parts.
Medical and Dental
Medical and dental tools such as forceps and implants, including replacement joints, screws, and plates, come with electroplated parts. This layer makes them more corrosion-resistant.
Prototyping
Since producing a prototype of custom or low-volume metal parts traditionally is quite expensive, manufacturers now combine the process with 3D printing when prototyping. That way, saving cost and time.
Power
Electroplating objects for electrical or solar power transmission helps improve their conductivity greatly. Besides, it improves their durability.
Jewelry
This is probably the industry with the highest application of this process. Manufacturers rely on this process to improve jewelry’s appearance and durability, such as bracelets, rings, pendants, etc.
Specific Examples of Electroplating Applications
- Use of chromium and zinc-nickel in electroplating bolts and fasteners used in the aerospace industry
- Chrome bumpers and other metal parts in the automotive industry
- Tooth inlays that aid dental procedures
- Silver and nickel plating of wires for improved conductivity
- Turning biodegradable items like flowers and bugs into works of art by artisans
RapidDirect’s Electroplating Services for Metal Parts

Every electroplating process requires electrolysis as its base. However, what differentiates one electroplating process from another is the solution employed during the process as well as the expertise of the professional. If need metal parts with electroplating finishing, RapidDirect is your best bet.
To ensure your product stands out among competitors, RapidDirect applies a top-quality surface finish with exquisite materials meeting your requirements. Besides, we use high texturization standards to ensure a quality finish for metallic parts. Also, since we consider time an important factor in production, we shorten the production process using our self-owned factory and its established networks.
At RapidDirect, our manufacturing ability is versatile and aids in the production of high precision and top-notch metal parts, ranging from CNC machining machines to sheet metal fabrication services. Our sheet metal fabrication aids in the production of high precision and top-notch metal parts.
Besides, our strict quality assurance ensures you get high-quality metal parts with outstanding finishing and aesthetics. What’s more, you can get a quote for your electroplating, and other manufacturing needs easily by visiting our online platform.
Conclusion
The electroplating process is one technology that has been around for a long time. It aids in the production of parts that have improved properties and are durable and aesthetically pleasing. For electroplating to be successful, it requires an anode, cathode, electrolyte, and power source.
Are you looking to electroplate an object? Doing so without proper guidance or training is a risky affair. So, it is best you visit RapidDirect for your electroplating needs.
FAQs
Yes, it is possible to electroplate plastics. However, you cannot achieve this by immersing the plastic material in an electrolyte. This is because it requires a special plating technique.
The main difference between them is that electroforming results in the creation of a new object while electroplating deposits a layer on an existing object called the substrate.
It occurs through a process called electrodeposition. The plating metal is the anode, while the other metal or substrate is the cathode. Introducing electric charge through the anode results in the oxidation of the plating metal. The electric current in the setup carries and deposits this oxidized metal into the cathode.